The Role of Securement Devices
After a vascular access device (VAD) has been inserted, catheter securement is one of the most important aspects of care and maintenance.1 The use of sutureless, adhesive securement devices in vascular access has become recommended as best practice because such devices are a cost-effective, reliable solution.1
Devices used to access the vascular system include, but are not limited to:1
- Peripheral intravenous (IV) cannulas
- Peripherally inserted central catheters (PICCs)
- Acute central venous catheters (CVCs)
There are also many other types of medical devices that require securement, for example:
- Nasogastric tube (NG tube) ― a thin, flexible tube inserted via the nose into the stomach; used to deliver or remove a variety of substances
- Foley catheter ― a thin, flexible tube inserted via the urethra into the bladder and prevented from slipping out by inflating a small balloon near the tip of the tube with liquid; used to drain urine
Note that catheter securement, sometimes referred to as catheter stabilization, can be divided into primary and secondary securement.2 Both are equally important.2 Primary catheter securement directly holds the catheter in place on the skin; secondary securement functions as a shock absorber to reduce any force the primary securement receives when energy is applied to the tubing by accident or rapid patient movement.2 Proper primary and secondary securement can reduce complications, increase patient comfort, and save money.2
“Since we adopted a non-suturing policy [for CVC securement] in our ICU, we have seen… a complete elimination of inadvertent needle sticks to the staff and no increase in inadvertent catheter removal.”
– Rihard Knafelj, MD, PhD, Senior ICU Consultant (Medical ICU, University Medical Center, Ljubljana, Slovenia)
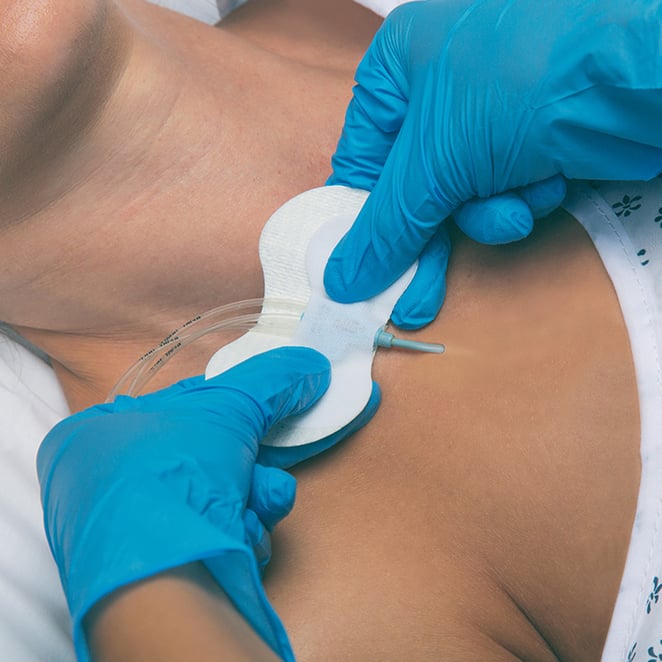
An Emphasis on Patient Comfort, Patient Safety
Grip-Lok® Securement Devices, manufactured by TIDI Products, are used to provide primary and secondary securement for tubes, lines, catheters, drains, hubs, and ports.
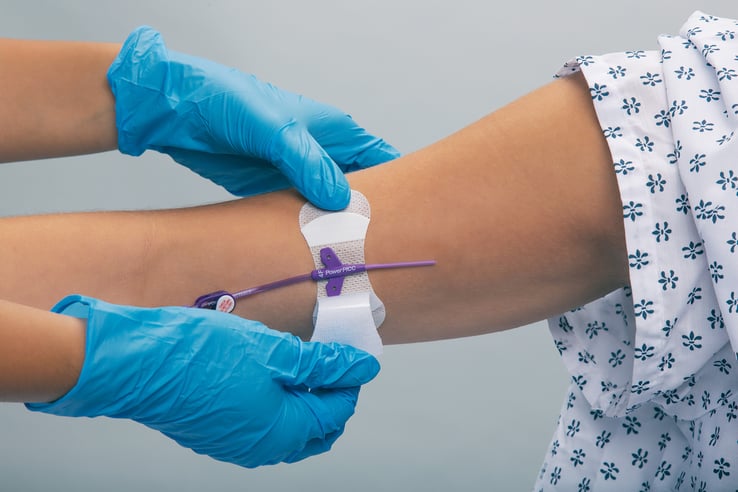
Optimized for patient comfort and safety, Grip-Lok devices offer numerous benefits, including:
- Medical-grade, hypoallergenic adhesive that promotes skin integrity
- Hook-and-loop design that allows repositioning and access to catheter after securement
- Made without latex and packaged sterile (can be used near insertion site or within sterile field)
- Can be easily placed and adjusted with gloved hands
- Helps prevent dislodgment and migration (reduces risk of lost lines and patient discomfort)
- Adheres to silicone, PVC, and other plastic compounds
- Meets INS guidelines for engineered securement3
- Grip-Lok adhesion is up to 4.9x stronger than commonly used tape4
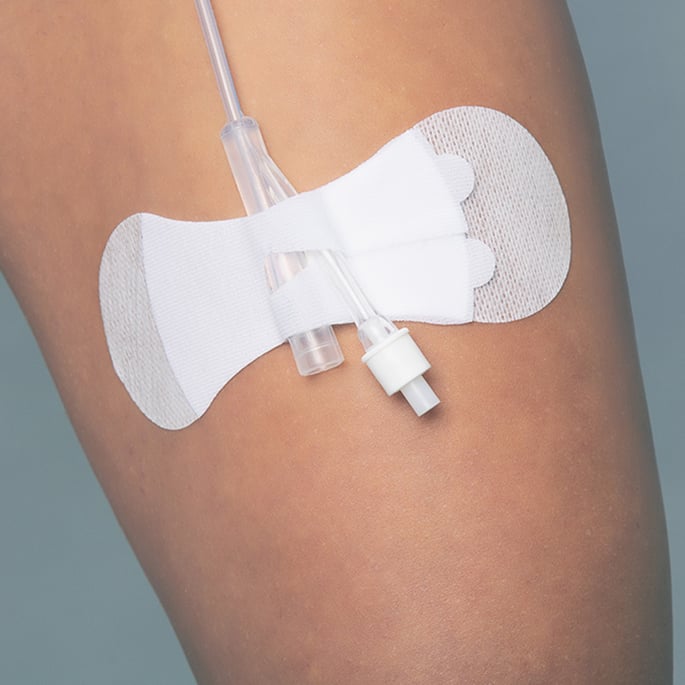
A low-profile design keeps the Grip-Lok device close to the skin while promoting patient comfort. There is no bulky, hard plastic mechanism that can create small areas of dead space.1 (Once covered by a film dressing, areas of dead space can trap moisture, which can lead to microbial colonization.1,5) Grip-Lok devices create a smooth, flat platform so the IV dressing can cover the entire catheter hub, ensuring that the exit site is completely covered with the film dressing.1
The Versatile Grip-Lok Product Series
For more than 20 years, medical professionals have relied on field-proven Grip-Lok Securement Devices. Made in the USA by TIDI Products, these securement solutions are used in many different healthcare settings, including hospitals, ambulatory surgery centers (ASCs), clinics, and doctor’s offices. They are also used in home healthcare.
Click here for details about the complete series of Grip-Lok securement products.
SOURCES
1 Barton A. Universal adhesive vascular access securement with Grip-Lok devices. Br J Nurs. 2020 Apr 23;29(8):S28-S33. doi: 10.12968/bjon.2020.29.8.S28. PMID: 32324466.
2 Czajka C, Frey AM, Schears G. Vascular access device stabilization and line securement. Am Nurse Today. 2018 Dec 13;13(12):22-24.
3 Infusion Nurses Society. Infusion Therapy Standards of Practice. J Infus Nurs. 2016;39(1S):S73.
4 Data on file at TIDI Products, LLC.
5 Rippon MG, Ousey K, Cutting KF. Wound healing and hyper-hydration: a counterintuitive model. J Wound Care. 2016 Feb;25(2):68, 70-5. doi: 10.12968/jowc.2016.25.2.68. PMID: 26878298.
