Ambulatory Surgery Centers (ASCs)
Running an efficient, fiscally responsible ambulatory surgery center, one dedicated to achieving the best possible clinical and patient outcomes, involves a multitude of ever-evolving challenges. The cost of the equipment, tools, robotic advancements, and medical devices associated with performing surgeries, for example, is an ongoing concern. And because most of these items carry the same cost for ASCs as for hospitals, it’s important to work with suppliers that provide the greatest value for the investment.
There are currently more than 6,000 ambulatory surgery centers in the United States, according to data provided by the Centers for Medicare & Medicaid Services (CMS).1-3 The following is a list of the leading specialties for Medicare-certified ASCs:1,3
- Orthopaedic
- Pain
- Ophthalmology
- Endoscopy
- Plastic
- Podiatry
- Otolaryngology
- Obstetrics/Gynecology
- Dental
TIDI Products is committed to offering medical devices that can help improve the patient-provider experience, optimize staff workflow, and reduce cost of care for ASCs, regardless of specialty.
Four Standardized Medical Product Solutions for ASCs
Each of these standardized medical product solutions is intended for use either before, during, or after surgery, as indicated:
- The patented C-Armor® Drape (shown below) is an easy-to-deploy, easy-to-retract protective barrier designed to maintain the integrity of the sterile surgical field during procedures with multiple C-arm swings. Only one C-Armor Drape is typically required per case.

- The Sterile-Z® Patient Drape (shown below) is an easy-to-use, transparent barrier designed to cover the sterile field and protect the intraoperative patient during 3D imaging. Unique Z-fold technology provides a simple method of drape separation that enables a removal process compliant with sterile technique guidelines published by AORN.4
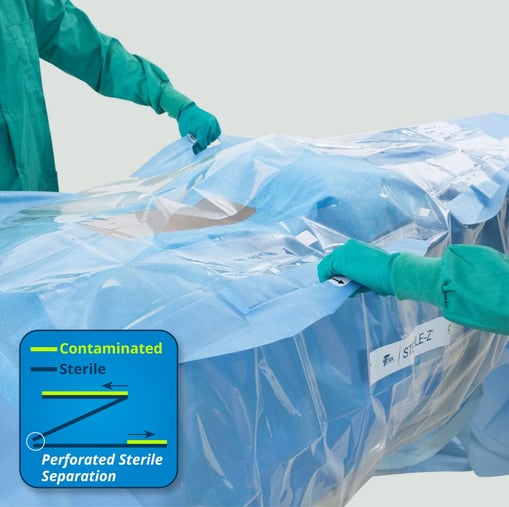
- The Sterile-Z® Back Table Cover is an easy-to-use, transparent barrier designed to maintain the sterility of operating room back tables when instruments and/or implants are not in use. Unique Z-fold technology enables a removal process compliant with sterile technique guidelines published by AORN.4 This cover lets staff quickly and efficiently protect sterile fields during procedural delays, potentially freeing up sufficient time to perform additional tasks. (Sterile-Z® Mayo Stand Cover also available.)
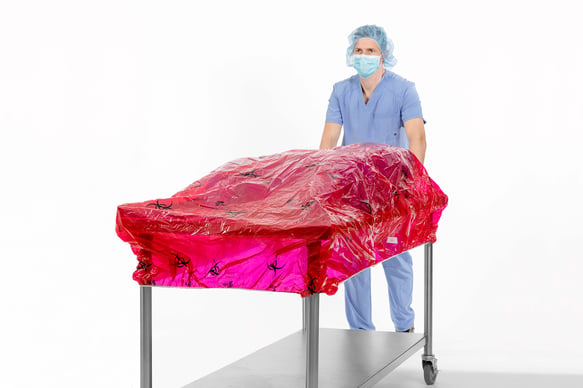
- The TIDIShield® Transport Cover (shown below) is a post-case back table containment cover designed to be used during the transport of biohazardous material from the surgical suite to decontamination. Available in five sizes, this impermeable red cover with biohazard symbols complies with guidelines published by AORN,5 OSHA,6 and AST7 for identifying biohazardous material during transport.
Additional Medical Products for ASCs
In addition to the medical devices listed above, TIDI Products offers the following items for ASCs:
- TIDIShield® Grab ‘n Go™ Protective Eye Shields are a preassembled, disposable form of critical PPE preloaded and shipped in a gravity-fed, tower-style dispenser to help busy ASC staff avoid wasting time locating, putting together, and cleaning eyewear prior to use. (Grab ‘n Go™ Face Shields also available.)
- PenBlade® Safety Scalpels help keep ASC personnel safe from sharps injuries. An easy pen-click motion that requires just one hand is used to fully retract the stainless steel blade.
- TIDI® Equipment Covers are designed to reduce contamination risk. Offerings include band/dome bags, C-arm covers, cassette covers, footswitch covers, probe covers, and urology bags.
TIDI Products offers items such as patient gowns, patient capes, patient drape sheets, and patient bibs as well.
Meeting ASC Challenges
Since last year, the number of Medicare-certified ASCs has increased from 6,109 to 6,223.2,3 The growing popularity of ASCs means facility administrators must continually look for new ways to keep pace with robust demand while maintaining quality of care and containing costs.
We can help! Click TIDI’s innovative medical products for ambulatory surgery centers to learn more.
SOURCES
1 Ambulatory Surgery Center Association. What is an ASC? / Specialties Served in ASCs. / Find an ASC Near You. https://www.ascassociation.org/about-ascs/surgery-centers. Last accessed online October 13, 2023.
2 Outpatient Surgery Magazine, A Division of AORN. What’s the Current ASC Count Nationwide? By Outpatient Surgery Editors. Published August 21, 2023. https://www.aorn.org/outpatient-surgery/maket-trends/article/what-s-the-current-asc-count-nationwide. Last accessed online October 13, 2023.
3 Centers for Medicare & Medicaid Services (CMS). https://www.cms.gov/
4 Guideline for sterile technique. In: Guidelines for Perioperative Practice. Denver, CO: AORN, Inc; 2019: 958, 4.2. (AORN does not endorse any company’s products or services.)
5 Perioperative Standards and Recommended Practices. 2018 Edition; Publisher: AORN, Inc. Tech IV.A. (AORN does not endorse any company’s products or services.)
6 Occupational Safety and Health Administration. (1970). Occupational safety and health standards: Bloodborne pathogens (OSHA Standard No. 1910.1030). United States Department of Labor. https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.1030
7 AST Guidelines: Standards of Practice for Decontamination of Surgical Instruments. https://www.ast.org/uploadedFiles/Main_Site/Content/About_Us/Standard_Decontamination_%20Surgical_Instruments_.pdf
